
Kazakh President Kassym-Jomart Tokayev addressing the OIC Summit on Science and Technology.
Kazakhstan will provide its QazVac vaccine to the member states of the Organisation of Islamic Cooperation (OIC) and consider its production in other OIC nations to boost cooperation in science, technology and innovation within the organisation, said Kazakh President Kassym-Jomart Tokayev at the second OIC Summit on Science and Technology held on June 16, 2021 via videoconference, reported Akorda.
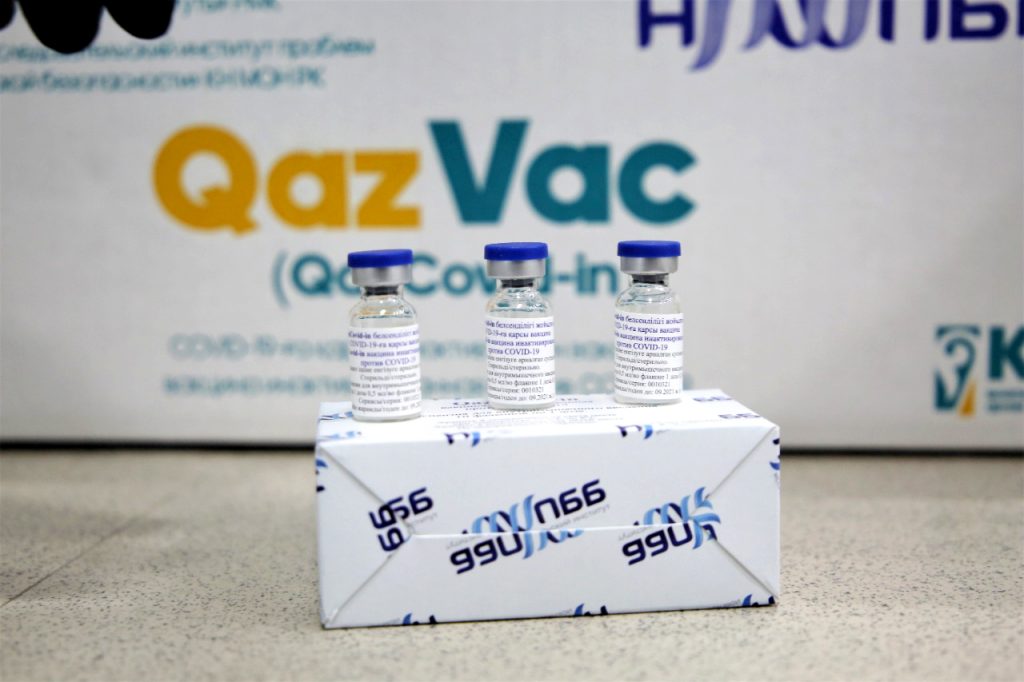 Kazakhstan is currently seeking the World Health Organisation’s (WHO) approval for the QazVac vaccine.
Kazakhstan is currently seeking the World Health Organisation’s (WHO) approval for the QazVac vaccine.
On May 28, WHO Director-General Dr Tedros Adhanom Ghebreyesus praised Kazakhstan’s efforts to curb Covid-19 infection in the country and its vaccine rollout during a virtual meeting with Mr Tokayev.
Till May 31, more than 2.1 million people in Kazakhstan had received at least one dose of the vaccine and 1.05 million were fully immunised against the virus.
“Two million vaccinated people is a very significant figure, many countries have not yet reached this level. Your ambitious goal to have 55 per cent of the country’s population vaccinated by the end of the year deserves respect,” said Dr Ghebreyesus.
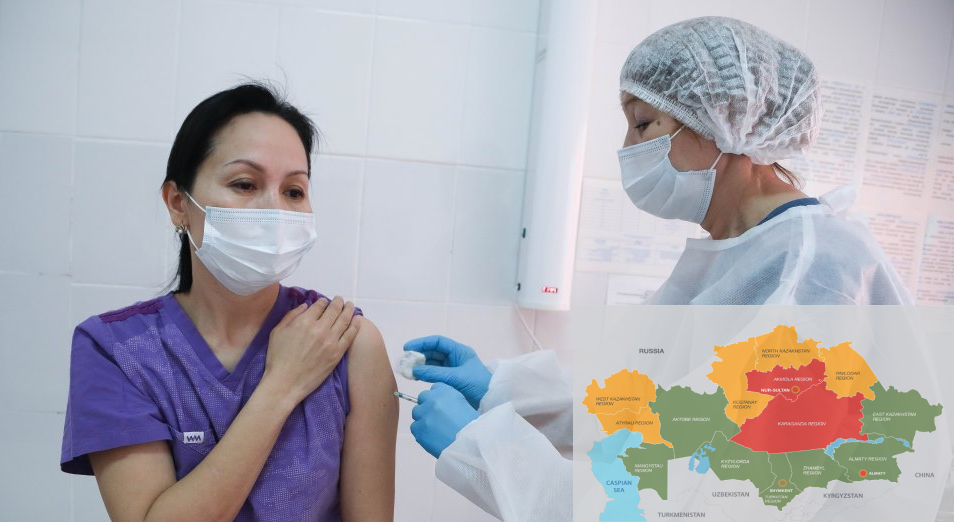 During the meeting, the prospects of strengthening cooperation between Kazakhstan and WHO in countering the pandemic were discussed. Mr Tokayev commended the level of cooperation between Kazakhstan and WHO.
During the meeting, the prospects of strengthening cooperation between Kazakhstan and WHO in countering the pandemic were discussed. Mr Tokayev commended the level of cooperation between Kazakhstan and WHO.
The Kazakh President also welcomed Mr Ghebreyesus’s opening remarks at the World Health Assembly, where he called for increased global efforts in getting people vaccinated against Covid-19, so that by September 2021 at least 10 per cent of the world’s population will be vaccinated and 30 per cent by the end of the year.
Mr Tokayev thanked WHO for providing Kazakhstan with protective and medical equipment during the initial, difficult days of the outbreak and briefed the WHO Director-General about the measures being taken by Kazakhstan to tackle the infections.
Dr Ghebreyesus praised Kazakhstan’s efforts to curb the spread of the virus.
The meeting also explored the development of the Primary Health Care policy and in particular the promotion of the 2018 Astana Declaration on Primary Health Care.
Mr Tokayev asked the WHO Director-General to support the Kazakh initiative to draft a Political Resolution on Primary Health Care, which is likely to be adopted at the United Nations General Assembly in 2022. The resolution includes the commitments of the Astana Declaration and Operational Framework on Primary Health Care.
Mr Tokayev also suggested that Dr Ghebreyesus implement a WHO One Health pilot project in Kazakhstan to improve the capacity building for Central Asia’s experts with an eye to creating a Regional Centre for Disease Control and Biosafety.
Meanwhile, the clinical trials of QazCoVac-P, another Kazakh vaccine against Covid-19, were launched on June 15 at the Kazakh Biosafety Research Institute, reported the institute’s press service.
QazCoVac-P is the Biosafety Research Institute’s second vaccine that has passed pre-clinical trials, held by the Kazakh Ministry of Healthcare. QazVac (QazCovid-in) passed the safety requirements earlier and the first batch of the vaccine was rolled out on April 22.
The clinical trials involve volunteers from the age group 18 to 50 and are held at the multidisciplinary hospital in Taraz.
While QazVac is an inactivated vaccine (where the virus is inactivated during the process of making the vaccine), QazCoVac-P is a subunit vaccine (composed of protein or glycoprotein components of a pathogen that are capable of inducing a protective immune response and may be produced by conventional biochemical or recombinant DNA technologies) based on artificially synthesisedproteins of the SARS-CoV-2 coronavirus.
 Subunit vaccines, similar to inactivated vaccines, do not contain live components of the virus and are considered safe. The adjuvant (a substance which enhances the body’s immune response to an antigen) contained in the vaccine effectively stimulates the immune response without adversely affecting a vaccinated person.
Subunit vaccines, similar to inactivated vaccines, do not contain live components of the virus and are considered safe. The adjuvant (a substance which enhances the body’s immune response to an antigen) contained in the vaccine effectively stimulates the immune response without adversely affecting a vaccinated person.
Since a subunit vaccine contains only the necessary antigens and does not include all the other constituents of the virus, side effects are less common. For example, vaccines against the flu, hepatitis B, pneumococcal, meningococcal and hemophilic infections are all subunit vaccines.
QazCoVac-P is also a two-dose vaccine. It stimulates immunity in laboratory animals on the 14th day after the intramuscular injection of the second dose.
Currently, Kazakhstan uses Russia’s Sputnik V, the locally produced QazVac and China’s Sinopharm, which is produced in the United Arab Emirates and named Hayat-Vax.







